What is “Functional Restoration”?
When imagining a treatment for spinal cord injury, it seems logical that repairing the damaged spinal cord is the answer. After all, that’s what’s “broken”, right? But a spinal cord injury leaves people with many functional losses. These can include losses of standing and walking; bladder or bowel control; postural control and balance; effective breathing; body temperature regulation; and more.
Functional restoration focuses on treating individual lost functions.
Through a technique called neuromodulation, a person with SCI can control neural pathways below the level of injury allowing for restoration of specific functions. Over the past three decades, researchers at the Cleveland FES Center have determined how to activate the neural pathways that provide hand grasp, respiratory assistance, trunk stability / postural control, bladder voiding and continence, bowel continence, standing, and stepping.
Up to now, these technologies have been available only to research subjects. The Institute for Functional Restoration is striving to make these solutions available to the wider SCI population by building them on the Network Neuroprosthesis platform and commercializing them. This means that not only will the broader population have an opportunity to benefit from these research advances, but they will also have the ability to have multiple functions restored.
MORE READING
A comprehensive list of peer-reviewed publications can be found on the FES Center website.
What is this technology?
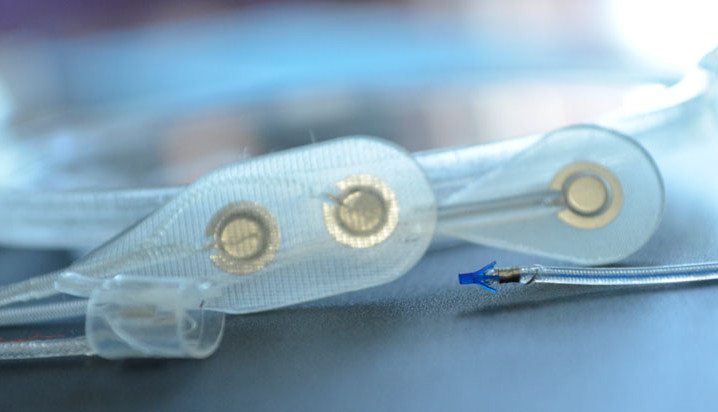
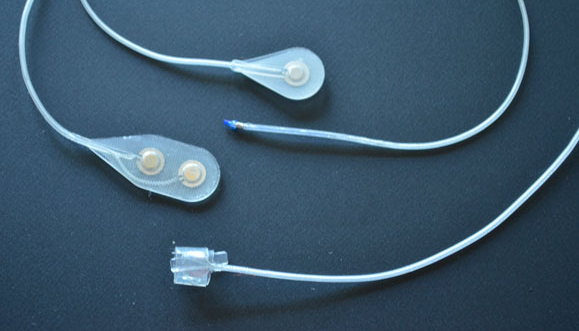
The Networked Neuroprosthesis is like a network computer inside the body. A rechargeable battery sends both power and communication to remote modules, which then send electrical pulses to muscles, causing their activation. By coordinating the activation of many muscles, functional movements can be restored.
People with spinal cord injury are missing multiple functions. They shouldn’t have to choose between having either hand function or trunk stability. They deserve to have multiple functions restored. The Networked Neuroprosthesis was designed specifically to meet the multiple functional needs of people with spinal cord injury.
When Can I Get This?
We are nearing the finish line!
After 15 years and $15M in federal funding our third-generation technology, the Networked Neuroprosthesis for hand function and trunk stability, is available to a limited number of research subjects taking part in an FDA-approved early feasibility clinical trial.
In 2020 we will initiate our pivotal clinical trial targeting hand function and expand to include participants at 2-3 other centers. Our goal is to complete that trial by 2022 and obtain FDA approval for the hand version of the NNP system. FDA approval will put the hand system on the market and enable people to seek it as a clinical treatment (no longer restricted to research trials).
Meanwhile, through our “Pipeline” we are continuing to finalize the systems that will restore other functions and get those clinical trials underway so that we can commercialize them as quickly as possible.
Click here to see the history of our development and how we are progressing on all fronts.
Click here to learn more about our clinical trial for hand and trunk function.
Who is the IFR Team?
Hunter Peckham is widely recognized as the leading expert in the field of neuroprosthetics through his extensive experience in the development of implantable neuroprostheses beginning in 1972, investigating the fundamental aspects of muscle fatigue and muscle metabolic changes, developing stimulation and control techniques for clinical applications, and deploying implantable systems into clinical trials and early commercialization.
Megan Moynahan sets the strategic direction for the IFR, establishing partnerships with key stakeholders within industry, foundations, and donors. She works to establish favorable regulatory and reimbursement policies and practices for neuromodulation systems intended to address the needs of orphan markets. She previously served as the Associate Director for Technology and Innovation at the US Food and Drug Administration’s Center for Devices and Radiological Health.
Michael Keith originated and refined the surgical implantation technique for implantable functional electrical stimulation (FES) systems for hand grasp, integrating it with surgical reconstruction of the hand and upper extremity to optimize functional control. He maintains research interests in the area of restoration of hand and upper extremity function to people with paralysis, has worked with American Academy of Orthopedic Surgeons to establish clinical practice guidelines through evidence-based outcome measures, and advocates for health policy.
Kevin Kilgore’s primary research interest is in the application of functional electrical stimulation (FES) to provide individuals with disabilities with increased independence and improved quality of life. His work has focused on FES as applied to cervical level SCI: these individuals have paralyzed muscles in both their hands and arms, and FES can be used to produce grasp and release motions, allowing them to perform many tasks independently. He has been a chief architect of the Networked Neuroprosthesis and continues to explore novel applications of FES, including various methods of electrically blocking nerve conduction to reduce spasticity or block pain.
John Chae’s research interests are in the areas of post-stroke, upper and lower limb motor and functional restoration, and shoulder pain in hemiplegia. His active projects include the investigation of: various forms of electrical stimulation for upper limb motor relearning; peroneal nerve stimulation for motor relearning and neuroprosthetic effects; implanted neuroprostheses for upper and lower limb motor restoration; & implanted and surface stimulation for reduction of shoulder pain.
Harry Hoyen’s research interests include clinical investigations in shoulder control reanimation in stroke, paralysis, and other biomechanical deficits, trunk control for paralytic disorders such as spinal cord injury, and treatment of spastic and paralytic hand and wrist problems.
Brian’s experience designing neuroprosthetics spans the 30-year history of Cleveland’s FES program, serving as the chief designer of the first neuroprosthetic hand system implanted in pioneer Jim Jatich. As research demands grew for neuroprosthetics, Brian served as the lead designer of second-generation FES systems featuring bi-directional communication, and oversaw technicians and students in the development of implanted sensor control methods. Brian holds numerous patents, has co-authored peer-review publications, regularly presents guest lectures, and continues to mentor students and young engineers. Brian currently leads the NNP Engineering Team in the design, development, evaluation, and prototype manufacturing of the Networked Neuroprosthesis.
Kim Anderson-Erisman helps Megan Moynahan operationalize the activities of the IFR. She has special expertise in communications and outreach as well as multi-site clinical trial operations. Kim is also internationally recognized as a leader in spinal cord injury research (SCI). Her research focuses on translational investigations and bridging the gap between basic science, clinical science, and the public community living with SCI. She has expertise in SCI outcome measures and has conducted multiple multi-center clinical trials. She was part of the leadership team running the 6 FDA-regulated Schwann cell transplantation clinical trials while a faculty member at the University of Miami. In Cleveland she is furthering her independent research efforts addressing issues important to people living with SCI.
As a Research Manager with training as a certified and licensed Occupational Therapist, Anne is deeply committed to improving the lives of people with spinal cord injury. Anne has grown her career by developing projects driven by the clinical needs of people with spinal cord injury. She currently co-manages a five-year multi-million dollar NIH project to restore function in people with spinal cord injury. In 2015, Anne received the Neilsen Foundation Allied Health Professional Research Award to develop an assessment for measuring lower motor neuron damage in the upper extremities of people with cervical level SCI.
Why is this Challenging?
|
‘When great technologies fail to achieve market success, it’s the patients who lose out.’ – Megan Moynahan, Executive Director, Institute for Functional Restoration. TEDxBrussels, 2013.
|
Believe it or not, the hard part of our mission is not the technology; it’s the business model. We’ve demonstrated we can give people the use of their hands again, bladder control, respiratory assistance and other functions. And we’re confident we can manufacture our technology and navigate all the regulatory requirements. But we have learned painful truths from our own history about the challenges of keeping a commercial enterprise going for the small market of spinal cord injury. This time around, our focus is on creating a sustainable commercial enterprise that will restore function to people with paralysis long into the future. |
How Can I Help?
We’re fueled by grants and philanthropy. You can help by getting the word out, volunteering or donating.